We are dedicated to improving the quality of essential medicines in low- and middle-income countries by strengthening regulatory systems and building capacity to monitor the quality of medicines throughout the supply chain.
Our unique approach is:
Holistic
From addressing quality-related aspects of medicine production and ensuring that quality is maintained until patient use, we aim to strengthen and improve the systems, structures, and processes that promote product quality.
Systems-based and sustainable
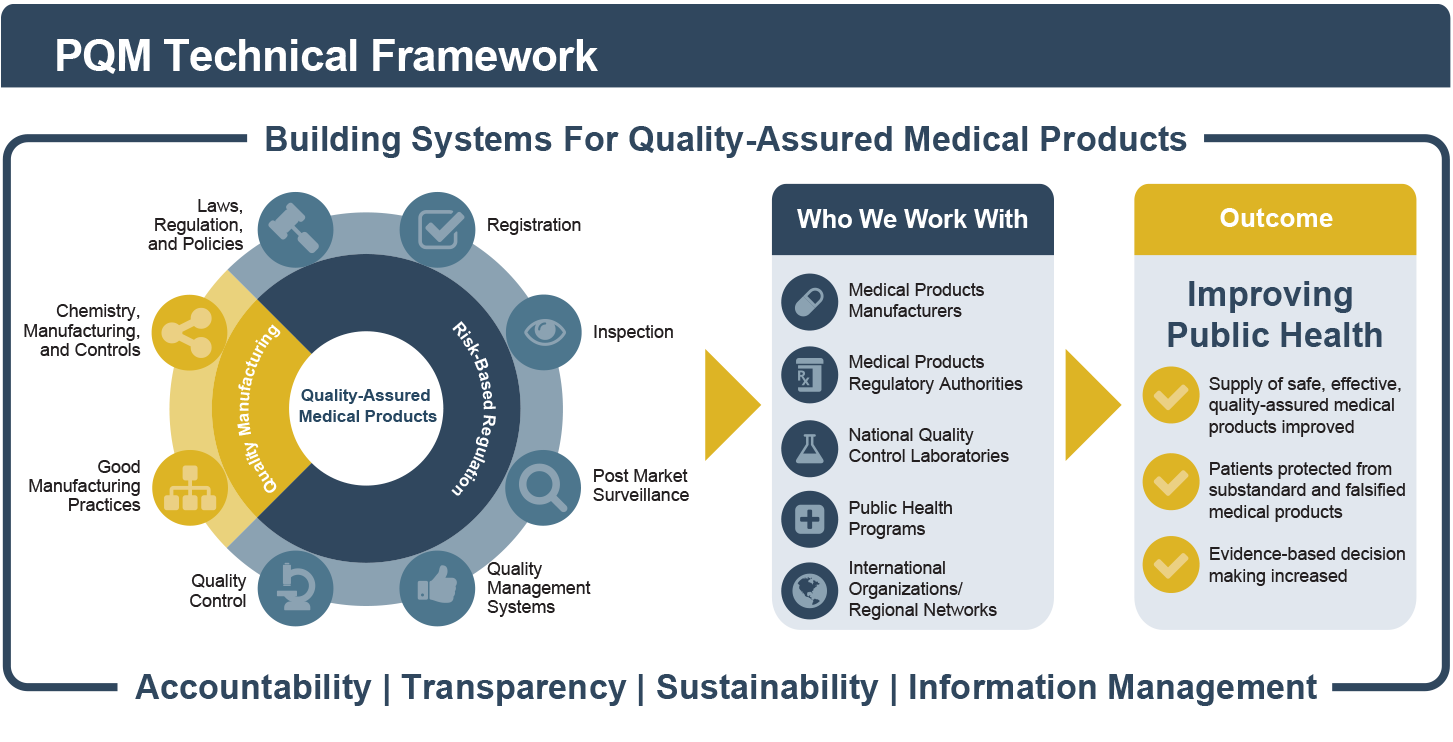
Recognizing the dynamic and cross-cutting relationships among different components of the health system, PQM seeks to address product quality issues in a sustainable manner using systems-based thinking and solutions. The framework below illustrates how we build quality assurance systems for medicines by working with key stakeholders in the areas of quality manufacturing and risk-based regulation to achieve critical public health outcomes.
Risk-based and pragmatic
PQM helps assess local risks to public health to prioritize interventions and direct human and financial resources where they are needed most. A risk-based approach optimizes the use of resources and enables countries to transition from donor-supported activities to locally funded and sustainable programs.
Focused on international standards
PQM helps countries build on their existing systems to achieve internationally recognized standards for quality control laboratories and manufacturers, such as those required to achieve ISO 17025:2005 accreditation or WHO prequalification.
Collaborative
PQM works with other implementing partners, multilateral organizations, government agencies, and academic institutions to coordinate efforts and maximize results. To address regulatory needs across multiple countries and facilitate South-to-South collaboration, PQM encourages harmonization at the regional level to help leverage resources.
Informed by nearly 200 years of experience
PQM and its partners benefit from USP’s expertise and 200 year history of improving health through public standards and related programs that help ensure the quality, safety, and benefit of medicines. For 25 years, USP and USAID have collaborated to achieve meaningful improvements in medicines quality in low- and middle-income countries around the world.
Related Links
- Learn more About PQM.
- Understand more about Our Work.
- Read PQM Success Stories.