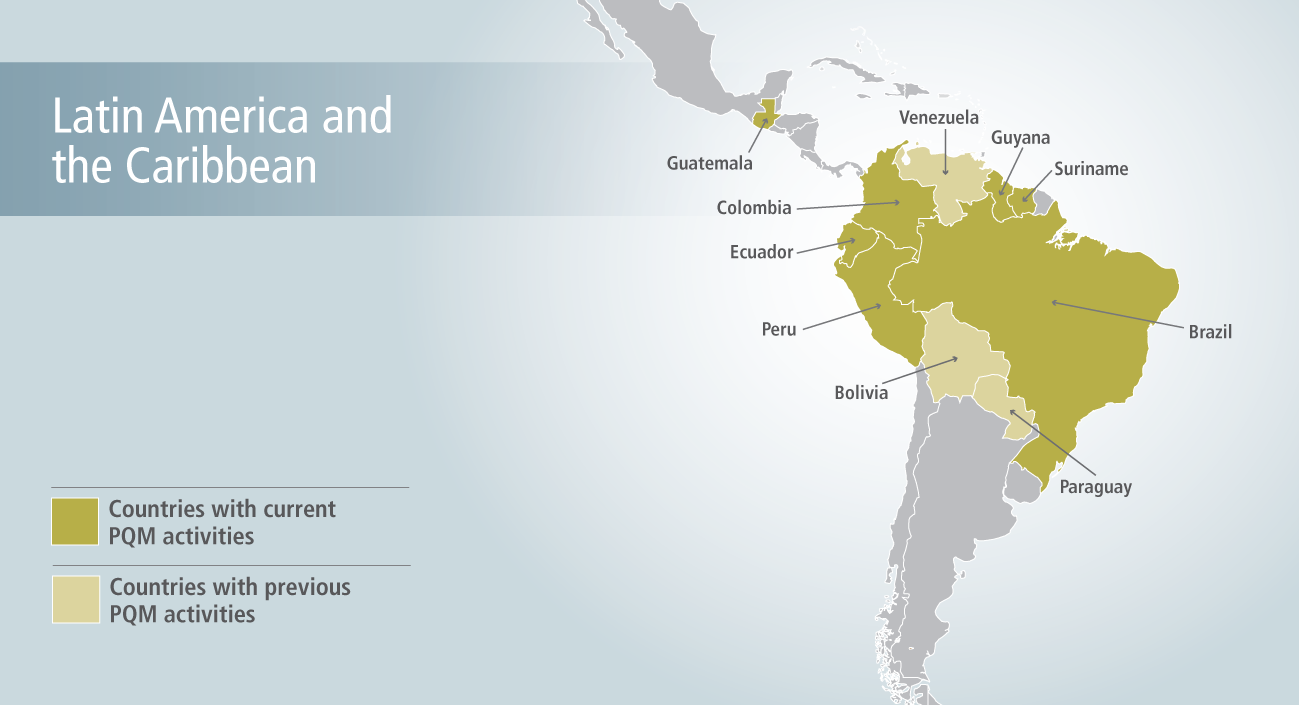
Promoting the Quality of Medicines (PQM) currently supports activities in 6 countries in Latin America and the Caribbean (LAC). Some of this work is performed through the Amazon Malaria Initiative (AMI) in collaboration with the Pan American Health Organization (PAHO), U.S. Centers for Disease Control and Prevention (CDC), and other partners.
Our Work in the Region
Strengthen National Regulatory Systems
- PQM sponsored a regional workshop in Lima, Peru that was attended by representatives from medicines regulatory authorities (MRAs), official medicines control laboratories (OMCLs), and selected schools of pharmacy from 16 LAC countries. The workshop served as a forum to explore mechanisms for establishing sustainable South-South collaborations to facilitate access to and maximize the use of regional resources for ensuring the quality of medicines in the region.
- PQM developed the "Three-Level Approach," a risk-based, rapid, and cost-effective approach for testing the quality of medicines. This approach allows countries with scarce resources to rapidly evaluate large volumes of medicines. To ensure the sustainability of this approach, PQM is collaborating with MRAs in LAC to incorporate the Three-Level Approach in their regulations, expanding its use beyond the field and throughout the supply chain, and developing methodologies to include more medicines that could be analyzed with screening tests.
- PQM previously conducted pilot studies in Guatemala and Peru of the quality of emergency obstetric and neonatal medicines that identified several failed products and revealed gaps in its quality assurance and quality control systems. In response to these findings, in Guatemala, PQM installed new drug registration software, trained the MRA staff on good practices for the inspection of manufacturers and storage facilities, supported implementation of the Three-Level Approach in decentralized health areas, and assisted the OMCL in attaining and expanding its ISO/IEC 17025:2005 accreditation.
- With PQM’s support, OMCLs of Bolivia and Peru have been added to the WHO prequalification list of quality control laboratories. These labs—among the first prequalified in Latin America—can now provide quality control services to United Nations agencies and organizations. The Peru OMCL has also achieved accreditation in ISO/IEC 17025:2005.
Increase Capacity to Detect Falsified, Substandard, and Unapproved Medical Products
- Amazon Malaria Initiative (AMI) countries provided routine data on antimalarial medicine quality until 2011. To address the lack of information since then, PQM surveyed the quality control activities performed on antimalarials in the 11-country AMI region in FY 2015-16; results show that only countries with strong regulatory systems performed quality control in a continuous and sustainable manner.
- Since FY 2015, Peru took total ownership of the Three-Level Approach and began implementing it without any PQM support; continuing with this, Peru’s national quality control lab expanded the assistance to regional health offices in performing Level 2 testing by incorporating additional academic institutions to the network of labs providing support.
- To support Level 1 assessment of physical and visual inspection in the field, PQM is developing an internet-based application that will be completed and deployed in FY 2017 in Peru and Ecuador. The Peru database contains over 250 products, including all antimalarials registered in the country as well as selected antibiotics and anti-inflammatory medicines.
Increase Evidence-Based Decision Making
- PQM conducted a study in the private and informal sectors of three malaria-endemic departments in Colombia which revealed antimalarial medicines being sold near public treatment centers where antimalarial medicines are provided free of charge. The study also identified falsified products, and very serious deficiencies in storage conditions.
- In two assessments previously performed in the private and informal sectors of Guyana and Suriname, PQM found a significant number of poor-quality antimalarials and the availability of therapies not compliant with national treatment guidelines. These studies also unveiled the possibility of diminished efficacy of artemisinin derivatives. PQM and AMI partners highlighted the risk to artemisinin efficacy posed by the quality and availability findings reported in the PQM assessments in an article in the Malaria Journal.
